Are you confident in your laboratory?
Published: 11th June 2019
How can you be sure that the laboratory you use supplies you accurate and reliable test results? - Test Method Validation
Sheffield Analytical Services (SAS) is a division of the Sheffield Assay Office, where precious metals have been analysed for over 240 years. SAS is now comprehensively equipped to offer an extremely wide range of analytical services to a diverse range of industry sectors such as jewellery trade, medical devices, automotive, aerospace, animal supplier and cosmetics. In all of these areas, we can assist by provision of an accurate and precise assay report. Test method validation together with continued test development are key factors to deliver the high standard of testing that our customers rightly expect.
Test methods are normally developed for an intended requirement so customers have confidence in the results produced by the test method. Method validation provides objective evidence that a test method is fit for purpose, meaning that the particular requirement for a specific use are fulfilled. The fitness for purpose includes an assessment and a balancing of technological possibilities, risks and costs.
This article provides a brief overview of the concepts and aspects to be considered when undertaking method validation.
Validation Parameters
The key parameters to consider in the validation process will depend on the nature of the test method and the range of samples types likely to be accounted. Recognised methods published by national or international organisations (ISO, ASTM etc.) have already been subject to rigorous validation during the collaborative process, and acceptance criteria are well established. An in-house developed method requires a more thorough validation process, and is often tailored to the individual requirements of the customer.
Some of most relevant parameters to consider in any validation process are:
Calibration linearity:
This is the ability to induce a signal (response) that is directly proportional to the concentration of the given analytical parameter. The calibration standard should be spaced over the concentration range of interest. Ideally, they must be prepared independently and not from aliquot of the same master solution.
Sensitivity:
Method sensitivity is the rate of change of the measured response with change in the concentration of analyte. A greater sensitivity usually means a lesser measurement uncertainty.
Accuracy:
Accuracy describes how close the result is to its true value and is influenced by random and systematic errors.
Precision:
This relates to the repeatability of the measurement. Precision is therefore a measure of spread of repeated measurement results and depends only on the distribution of random errors- it gives no indication of how close those results are to the true value. The difference between accuracy and precision can be seen here:
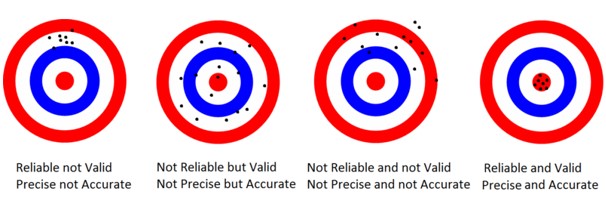
Repeatability:
The closeness of the agreement between the results of successive measurements which are carried out under the same conditions of measurement. These conditions include using the same measurement procedure, analyst, instrument and location, and are repeated over a short period of time.
Reproducibility:
The closeness of the agreement between the results of measurements which are carried out under changed conditions of measurement. The changes to conditions include using different analysts, different instruments, reference standards and locations, and are repeated over a longer period of time i.e. over several days or weeks.
Uncertainty:
This parameter characterizes the dispersion of the values that could reasonably be attributed to the analyte. Knowledge of uncertainty is necessary for effective comparison of measurement and for comparison of measurement with specification limits. Therefore the estimation of uncertainty may be considered an essential requirement of method validation.
Limit of detection:
It can be defined as the smallest amount or concentration of an analyte that can be reliably detected or differentiated from the background for a particular matrix. All matrix interferences must be taken into account when determine the detection limit. The detection limit may be determine by the analysis of a large number of blanks. The LOD is expressed as mean sample blank value plus three standards deviation. In addition the Limit of quantification is expressed as mean sample blank value plus ten times standards deviation or also three times the LOD.
There are a more parameters which may be considered during the validation process, however not all these parameters need to be assessed for all methods. The test validation should be sufficient to ensure that method satisfies the customer’s requirements.
Conclusion
Sheffield Analytical Services has strong experience in conducting the validation process, not only for its in-house analytical tests but also for helping its customers to validate their own specific testing requirements.
Don’t just take our word for it, our test methods are assessed regularly by the United Kingdom Accreditation Service (UKAS) to ensure they meet the strict requirements of the ISO/IEC 17025:2017 international standard.
If you require further information on method validation please contact us.
The Sheffield Assay Office was established in 1773, under an Act of Parliament and today the company assays and hallmarks the precious metals - silver, gold, platinum and palladium. Sheffield Assay Office is one of only four UK assay offices who all work to uphold the Hallmarking Act of 1973 and continue to ensure consumer protection for customers purchasing precious metals.
To find out more about the whole range of services offered by Sheffield Assay Office, such as our hallmarking and analytical services, please email us at info@assayoffice.co.uk or complete the contact form on our website at http://www.assayoffice.co.uk/contact-us,
Sign up here to all the latest news from Sheffield Assay Office direct to your inbox